SaeboVR Commercial Launch
Barron Associates, Inc. is pleased to announce the commercial launch of the SaeboVR product through its exclusive licensee Virtual Therapy Solutions, LLC under a marketing and distribution agreement with Saebo, Inc. (https://www.saebo.com/saebovr/). Saebo is currently offering free SaeboVR demos to qualified institutions. The SaeboVR product was developed under a Phase II SBIR grant (2R44HD071745-02) to Barron Associates, Inc. from the NIH’s National Institute for Child Health and Human Development (NICHD) under the title “Virtual Occupational Therapy Application (VOTA).” The VOTA Phase II SBIR effort included system development, successful clinical trials, and completion of FDA clearance actions.
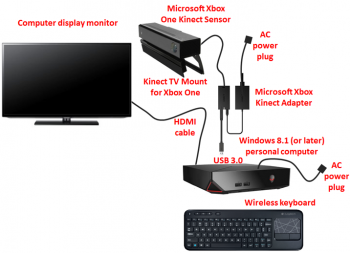
The SaeboVR software system, used with the Microsoft Kinect, is intended to be used to support repetitive task practice for rehabilitation of adults in a clinical or home setting consistent with Standard of Care for upper extremity (UE) rehabilitation. The system is based on simulated activities of daily living (ADLs) for the UE within a virtual world. VOTA uses a Microsoft Kinect Sensor to track patient shoulder and elbow movements, which are translated into equivalent movements of an avatar that represents the patient in a virtual environment.
Saebo’s European affiliate, SaeboUK is featuring SaeboVR at the RehabWeek expo in London July 17-21. (http://www.rehabweek.org).
For more information on SaeboVR, contact Saebo, Inc. (www.saebo.com, 888.284.5433).